Healthcare Human Factors Engineering
Human Factors Engineering focuses on designing products that match human capabilities and account for human limitations. This makes products easier to use, more effective, and safer. This is critical when designing medical products such as medical devices, combination products, and in-vitro diagnostics.
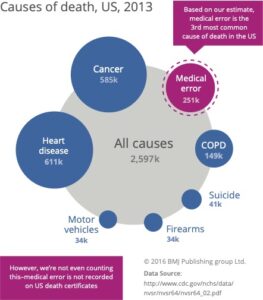
Products with inadequate human factors engineering cause use error — when users make mistakes due to bad design. These errors can cause complications, injury, or even death. Indeed, studies demonstrate that medical error is the third-leading cause of death in the United States. A significant number of these preventable deaths are caused by use error.

Use Error, Not User Error
Note the here: we use the term “use error” rather than “user error.” This terminology highlights that a design can make errors more likely by failing to consider the limitations of users. Further, it removes the shame and blame from the user who made the mistake. Typically, the error is not caused by incompetence or malice, but by a simple disconnect between user capabilities and design requirements.
FDA Human Factors Submissions
The FDA now requires manufacturers to provide Human Factors analyses in their regulatory submissions. These analyses demonstrate that the products can be used safely and effectively. What’s more, they apply not only to the product itself, but to packaging, labeling, training, and instructions as well.
The submissions describe the Human Factors Engineering activities throughout the entire design cycle. They further show how problems were identified and mitigated, and how the design improved. Addressing these components is no small feat, but with some smart planning, it is achievable.
Can’t we do this In House?
You might wonder, “Can’t we just do this in-house? After all, we have a regulatory consultant and a quality engineering group. Perhaps they can follow FDA’s Human Factors Guidance and save money on outside resources.” Of course, this sentiment is understandable. The expense of product development can tempt you to conduct Human Factors research on your own.
In fact, many of our clients thought this originally. Many clients come to us only after they have been rejected by the FDA the first time around. Unfortunately, many learned the hard way that this requirement should not be taken lightly.
Regulatory submission is not the time to take chances by pinching pennies. If Human Factors activities are conducted inappropriately, or the report is confusing, the FDA can delay or even prevent approval. They may request additional analyses or require additional usability testing. This is not only frustrating, but is also exceedingly expensive. During this time, you cannot sell your product directly, resulting in investors losing confidence and stock prices dropping.
FDA Human Factors Submission Failure Rate
The FDA Human Factors submissions record is atrocious, with fewer than 10% of those reviewed being successful on the first try. One FDA Human Factors reviewer put it plainly: “This is pretty sad, I can tell you.”
The causes of this low acceptance rate range from missing or incorrectly developed use-risk analysis to conducting the usability testing incorrectly. Specifically, there may be issues associated with the user groups, selection of research participants, or research procedure. Other times, there have been no usability activities done at all.
Although these results are disappointing, they are not surprising, as these requirements and analyses are still foreign to many companies. At Research Collective, our experience has been just the opposite. Throughout our dozens of submissions, we have never had an FDA Human Factors submission rejected.
Why Hire a Human Factors Expert
Recently, we interviewed several clients to understand why they prefer to hire a Human Factors Expert when seeking FDA medical product approval. We then categorized their responses to better comprehend the big picture. Here is what they told us.
Strategic Guidance
The most common reason for hiring a human factors expert had to do with the ability to provide strategic guidance. This guidance can help determine exactly which activities are required for your specific product and your specific situation.
Sometimes, for example, when there are no critical tasks, you do not need to conduct usability testing at all. You might still choose to do usability testing to improve your design, but it may not be required for the submission.
This is an important distinction. For example, one client reminded us about a Class 1 device project in which a regulatory consultant told him it required usability testing. We did a quick use-related risk analysis and found that no serious harm could result from misuse. As a result, usability testing was not necessary for the FDA submission. This discovery saved that client a sizable amount of money and time.
Efficiency
The second reason for hiring a human factors expert is efficiency. A specialized team with M.S. and Ph.D. degrees in Human Factors enables you to move quickly and with confidence. They have most likely seen the challenge you are experiencing several times over and know how to address it.
Recent Developments
In addition to this, human factors experts stay current on recent developments, concerns, and areas of focus within the FDA. Their training and experience prepare them to draft reports specifically directed to Human Factors Engineers, such as those at the FDA, by crafting word responses and formatting reports that highlight the most important issues.
In fact, one client, who previously served as an FDA reviewer, engaged our services recently. She contacted us because she found our “FDA submissions were so well organized and easy to read. They highlighted just the right information and answered just the right questions. I did not have to dig around to find what I was looking for.” That is what happens when Human Factors Experts write reports for other Human Factors experts.
Speed
The fourth reason is speed. An external human factors expert can simply do the work faster. One long-term client pointed out that a good external consultant can conduct twice the work in half the time of an internal resource. This is often because the external expert does not need to attend all-hands meetings, get pulled into politics, or conduct various kinds of company-required training.
The external resource has one job: to get work done. Because external Human Factors Engineers do this work all the time, they are fast. In many cases, an external human factors expert has developed dozens, if not hundreds, of use-risk analyses, user profiles, and environmental profiles throughout their career.
The resources available to an external Human Factors Engineer when conducting usability testing also enable work to be done more rapidly and efficiently. Some examples of this come in the form of recruiting, where experience conducting usability testing creates relationships with trusted vendors or allows for an extensive participant recruitment database to be accessed.
External human factors engineers also utilize their expertise in not so obvious ways, such as knowing the going compensation rate for participants depending on varying demographics or being knowledgeable of documentation required based on new legislation, such as the Sunshine Act. This speeds up the whole process.
Conclusion
At Research Collective, we work with companies to walk them through each step of the FDA submission process, making sure to highlight all of the benefits of giving us the responsibility to complete the process. Whether it be to save time, money, or stress, or to make sure your product is easy to use, we will be the Human Factors Experts who will utilize our years of knowledge and expertise to get your FDA submission approved.
Contact us to learn how our human factors engineering experts can help you expedite approval of your FDA submission.