Two nurses were caring for a 15-day-old baby with a congenital heart defect and a rapid heart rate. As is routine, they administered digoxin, a common drug for slowing heartbeats. Tragically, however, they made a mathematical error, giving 220 μg of digoxin rather than the intended 22 μg. The massive overdose caused the baby to go into cardiac arrest. He died a few days later.
The Problem
These were not incompetent healthcare providers. They were registered nurses with years of experience and strong performance records. They got into nursing for the right reason — to take care of people, infants, no less. Shaming or blaming them will not reduce the likelihood of this happening again. This was an error, pure and simple. And, it probably could have been prevented by applying human factors in healthcare during product development.
This problem, called “death by decimal,” illustrates the dangers of medical error in healthcare, which kills approximately 251,000 Americans each year. This makes medical error the third leading cause of death in the U.S., behind heart disease and cancer. It accounts for 9.5% of all U.S. deaths, and is like two 747 jumbo jets crashing every day. Worse, these numbers describe in-patient deaths only, not those that occur from errors in ambulatory settings, clinics, therapy, and home.
Death by decimal is by no means the only medical error. Other deaths have resulted from insulin pumps, ablation systems, automated external defibrillators, and duodenoscope reprocessing, for example. There are anesthesia-related complications, wrong site and wrong-patient surgery, medication errors, retained foreign objects, and surgical fires.
Medical Devices are Everywhere
Medical devices diagnose, prevent, monitor, treat, alleviate, or compensate for disease or injury (World Health Organization, 2020). They include everything from tongue depressors to robotic surgical systems. Indeed, more than 1,700 types of medical devices are used in every environment, from intensive care to patient bedrooms. Even public spaces like airports house automatic external defibrillators and first aid kits.
The proliferation of medical devices is likely to continue unabated for the next decade. Fortune Business Insights (2019), for example, projects the medical device industry to grow to $612.7 billion per year by 2025, largely due to advances in technology, trends in lifestyle-associated disease, and an aging population. Consequently, reducing the volume and severity of medical error is one of design’s greatest challenges.
Human Factors Engineering Can Help
When designed well, medical devices are invaluable to patients, healthcare providers, and caregivers. When designed poorly, however, they can cause harm or even death. This is the challenge of healthcare human factors engineering. Medical devices need to be designed for real people and need to take into consideration all their limitations and foibles. To that end, human factors experts apply scientific knowledge and human factors in healthcare to how people work, derived from anatomy, physiology, sensory and perceptual systems, cognition, emotion, social behavior, and motor control, to make medical products more useful, usable, desirable, and safe.
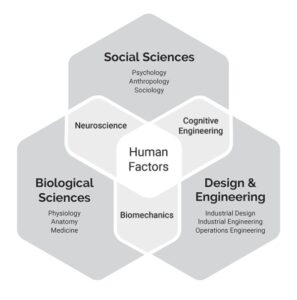
Human factors engineering relies on social and biological sciences such as psychology (cognitive science), anthropology, physiology, sociology, and medicine, to name a few. Healthcare human factors also rely on engineering and design disciplines, such as industrial engineering, biomedical engineering, industrial design, and mechanical engineering. This is illustrated in the figure below.
Goals of Human Factors Engineering in Healthcare
As mentioned above, successful medical devices have four interrelated qualities, illustrated below:
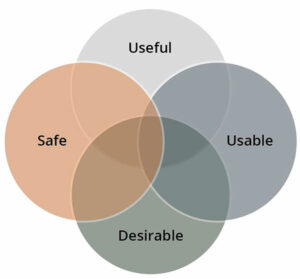
First, they are useful, meaning they enable the user to do something not easily accomplished without the device. Second, they are usable, providing the following:
- Easy to learn: The user can employ the device for its intended use quickly and easily without undue training
- Efficient to use: Once learned, the device has few extraneous steps or time lags
- Easy to remember: After a break from using the device, users can get up to speed with it again quickly
- Safe: It protects the user from making errors, but also enables the user to easily recover from any errors that are made
Third, they are desirable, eliciting emotions that are appropriate to the device’s use. People want to use such a device and are likely to choose it when deciding between it and its competitors. Fourth, and most important, they are safe. They protect users, patients, and others from undue harm.
The connection between the user and the device is the user interface (UI), defined as everything the user comes into contact with physically, perceptually, and cognitively. These components usually come in the form of hardware, software, packaging, websites, communications, labeling, instructions for use (IFU), and so on.
Benefits of Human Factors Expertise
In addition to making healthcare safer, healthcare human factors make good business sense.
Increased Sales
First, it can improve sales, since people prefer devices that are easy to use. This, of course, translates into an increased willingness to repurchase. Once people become familiar with your device, it erects a barrier to competition. This is advantageous because it is easier to retain customers than to attract them in the first place.
Improved Product Reviews
Second, strong applications of human factors in healthcare lead to strong product reviews. Nowadays, many publications and review websites are dedicated to product ease of use. Better usability yields better reviews.
Improved Brand Image
Third, human factors engineering leads to an improved brand image. Product reviews are repeated less formally through word-of-mouth discussions. People talk, and in healthcare, they have preferred instruments, devices, and products. The good ones are discussed positively while the bad ones are not. This is the grassroots reflection of your brand. Positive reviews and discussions bolster the brand, whereas negative ones kill it.
Increased Efficiency of Use
Fourth, easy-to-use devices improve task performance, making the task easier to execute, faster, and more consistent. These devices require less training time for users to become proficient, translating into improved financial performance. Efficiency increases profit.
Improve Patient Outcomes and Reduce Risk
Fifth, human factors engineering (HFE) can help improve patient outcomes and reduce product liability risk. For example, patients are more likely to be compliant when using these products because they involve less of a cognitive burden. In other words, HFE can facilitate correct and frequent use. Further, usable devices reduce the likelihood, frequency, and severity of use error. And when use errors do occur, usable devices facilitate recovery.
Facilitate Regulatory Approval
Finally, superior human factors engineering facilitates regulatory approval. Regulatory bodies such as the Food and Drug Administration (FDA) recognize that errors involving the misuse of medical devices are a considerable source of harm among patients and users. As a result, they require that many Class II and all Class III devices include human factors engineering activities, including use-risk analysis, identification of critical tasks, and usability testing (FDA, 2016).
We Can Help
Of course, the opposite occurs when HFE is ignored. Lack of HFE can reduce sales, decrease adoption of the device, satisfaction, and willingness to repurchase, and can result in more frequent and more dangerous use errors.
After reading all of the beneficial impacts and importance that human factors engineering serves in medical device development, you may be asking yourself, “How can I ensure that my medical device is truly safe and easy to use?” We at Research Collective can help answer that question. Whether you are looking to conduct initial formative testing or preparing for validation testing, we can be the human factors experts that utilize our knowledge and experience to guide you.
We take pride in our history of successful FDA submissions and helping companies bring products to market that are safe and easy to use. When planning for your medical device design, make sure to consider the human factors aspect and bring on a team that will help you every step of the way.
Contact us to learn how our human factors experts can help apply healthcare human factors best practices to ensure your medical devices are use-friendly and safe.